Chemical substances classified as drug precursors: what the regulation says
La regularización de sustancias precursoras de drogas ha supuesto siempre un debate entre los países en cuanto a su fabricación y comercialización. Esto es debido al empleo de dichas sustancias para fines ilícitos, como la formulación de estupefacientes.
Keep reading
Drug Precursors Regulated by the European Union: 2 New Substances in Delegated Regulation (EU) 2025/1475
Delegated Regulation (EU) 2025/1475 adds 2 new substances to the drug precursors regulated by the EU. Find out which ones!
Keep reading
REGULATION (EU) 2025/1731: UPDATE OF REACH ANNEX XVII WITH NEW CMR 1B SUBSTANCES
The EU updates REACH with Regulation (EU) 2025/1731, adding CMR 1B substances to Annex XVII. It enters into force on 1 September 2025.
Keep reading
Who Needs to Comply with ABNT NBR 14725:2023?
Learn how to comply with ABNT NBR 14725:2023 and ensure the classification, labeling, and SDS of chemical products in accordance with GHS and Brazilian legislation.
Keep reading
How the new ABNT NBR 14725:2023 standard impacts chemical product labelling in Brazil
Learn how ABNT NBR 14725:2023 affects chemical product labelling and what challenges companies face in adapting to the updated GHS standards.
Keep reading
Cosmetic Product Labelling: Commission Implementing Decision (EU) 2025/1175 and Regulatory Developments in Europe
Learn how EU Decision 2025/1175 affects the labelling of cosmetics, detergents, and aromatic products across Europe.
Keep reading
SVHC REACH List: New substances added by ECHA
ECHA has updated the SVHC list. Learn about the newly added substances and what companies must do to comply with the REACH regulation.
Keep reading
New Update to the CLP Regulation: Delegated Regulation (EU) 2025/1222 (ATP 23)
Discover the key changes in ATP 23 to CLP: new classifications, affected substances, and compliance deadlines under Regulation (EU) 2025/1222.
Keep reading
SDS Software: how eQgest can help you draft the document in line with current Brazilian regulations
As an SDS software, eQgest ensures compliance with the new ABNT NBR 14725:2023 regulation requirements
Keep reading
How to create an SDS according to ABNT NBR 14725:2023 to market in Brazil
Discover how the Safety Data Sheet (SDS) must be created according to the new Brazilian standard ABNT NBR 14725:2023
Keep reading.jpg)
Everything you need to know about the new ABNT NBR 14725 2023
Here you will find the important changes introduced by the new ABNT NBR 14725 2023, including the transition from FISPQ to SDS.
Keep reading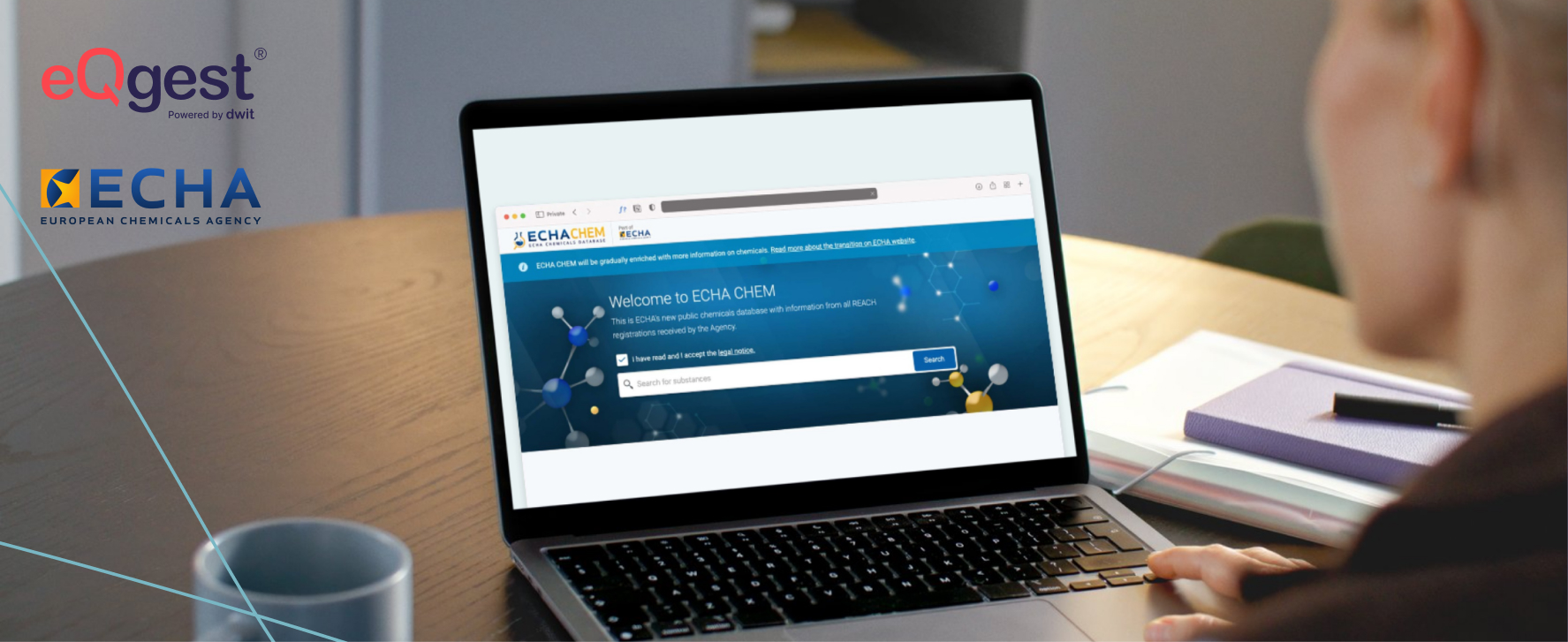
What is the ECHA database and why is it important for SDSs?
Discover how the ECHA database and the new ECHA CHEM facilitate the preparation of safety data sheets and regulatory compliance.
Keep reading
Annex VIII of the CLP: Harmonised Notification to Poison Centres
Find out what you need to know about CLP Annex VIII and the steps to follow to avoid legal risks for your company.
Keep reading
Do you know the difference between an EC and list number?
Discover what the EC number of chemical substances is, how it differs from the list number, and its role in REACH and CLP regulations.
Keep reading
Update of REACH Annex XVII: all about Regulation (EU) 2025/660
Find out about the latest changes to REACH Annex XVII under Regulation (EU) 2025/660.
Keep reading
How to properly manage the chemical labels
Knowing how to manage chemical labels is essential to ensure regulatory compliance - we show you how!
Keep reading
New CoRAP Draft 2025-2027
ECHA has published the updated draft CoRAP 2025-2027. Find out more about the substances included and removed.
Keep reading
ECHA Adds 5 SVHC Substances to the Candidate List
Find out which SVHC substances have been added by ECHA to the list and how they could impact your products
Keep reading
ADR 2025: Updates on the regulation of the transport of dangerous goods
Find out about the main updates introduced in the ADR 2025 on the transport of dangerous goods
Keep reading
REACH-EN-FORCE-11: Key Errors in Safety Data Sheets
Learn about the key eros in SDS highlighted in REACH-EN-FORCE-11 and how to comply with EU regulations
Keep reading
New Updates to the PIC Regulation in 2025
Learn about the latest updates to the PIC Regulation that directly affect the export of chemical products by EU companies.
Keep reading
IFRA 51, New Standards on the Safety of Fragrance Ingredients
Learn about the new norms established in the aromatic standards under the name of IFRA 51. Always comply with the legislation with eQgest
Keep reading
Precursors to Explosives: Harmonization Regulation for Their Commercialization and Use
Learn about the new harmonisation regulation on explosive precursors for their marketing and use. Don't miss it!
Keep reading-1731425414.png)
Moving Towards a Globally Harmonized System (GHS)
The GHS Globally Harmonized System of Classification and Labelling of Chemicals is the culmination of more than two decades of work.
Keep reading
UFI in chemical products: function and requirements
Discover everything you need to know about the UFI code and how to generate it correctly to comply with regulations
Keep reading
Tools for Risk Prevention: Safety Data Sheets
Importance of Safety Data Sheets (SDS) of chemical substances for risk prevention in chemical industries
Keep reading
EQGEST aporta una solución IT fiable y ágil a un gran grupo del sector de distribución de materias primas para la industria química
EQGEST aporta una solución IT fiable y ágil a un gran grupo del sector de distribución de materias primas para la industria química.
Keep reading

 + 34 900 802 588
+ 34 900 802 588 Client access
Client access

